Enantioselective addition of acetaldehyde to nitroalkenes constitutes a very interesting and cost-efficient route to access γ-amino acids. However, enamine-catalysed addition of acetaldehyde is extremely challenging; in fact, it could easily undergo self-condensation reactions.
Ground-breaking results were published by Hayashi and List in 2008. The protocols were not easily applicable on industrial scale to access key-intermediates to API; in fact, they suffered by the use of a large excess of acetaldehyde, a non-benign reagent. Pericàs, then, reported the use of paraldehyde and supported catalysts to circumevent the aforementioned problems. Nevertheless, they used 10equivalents of acetaldehyde and a relatively high catalyst loading of supported organocatalysts that, despite the potential recyclability, bring a considerable cost contribution to a manufacture process.
Giuliana and Valeria have now developed a cost-efficient protocol for the enantioselective addition of acetaldehyde to nitroalkenes. Besides using inexpensive reagents and an affordable organocatalyst, this newly developed approach, makes use of a masked acetaldehyde for a safer process, low catalyst loadings and doesn’t need a large excess of reagents.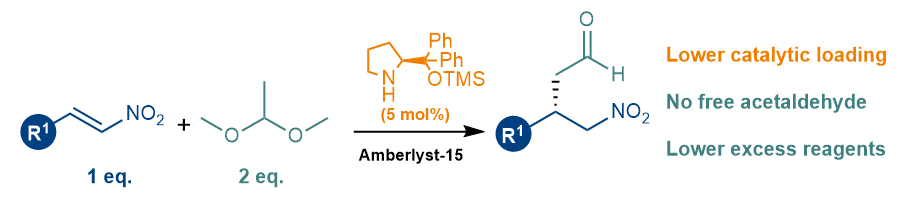 If you want to learn more, please follow this link or get in touch with us – in fact, we are working to improve this protocol to make it work in benign solvents.
If you want to learn more, please follow this link or get in touch with us – in fact, we are working to improve this protocol to make it work in benign solvents.

